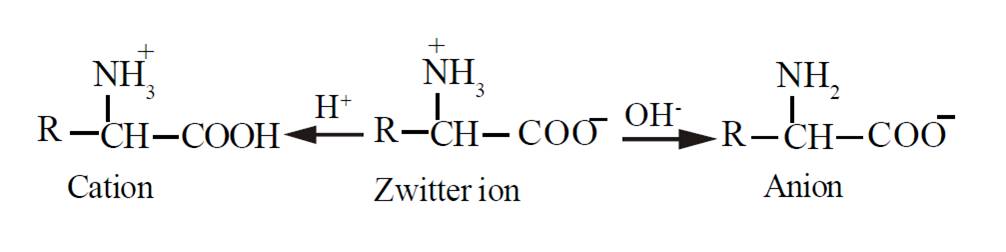ISC 11>Content>Unit-4>Biomolecules :PROTEINS
CLASS PRESENTATION
Proteins are the most abundant biological macromolecules, occurring in all cells and all parts of cells. Proteins also occur in great variety; thousands of different kinds, ranging in size from relatively small peptides to huge polymers with molecular weights in the millions,may be found in a single cell. Moreover, proteins exhibit enormous diversity of biological function and are the most important final products of the information pathways.
AMINO ACIDS (BUILDING BLOCKS OF PROTEINS )
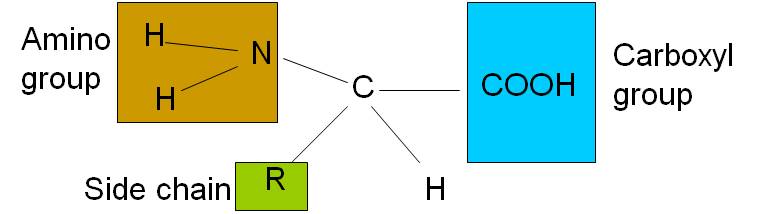
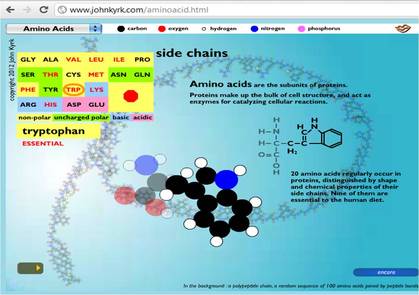
- Amino acids are the simplest units of a protein molecule and they form the building blocks of protein structure.
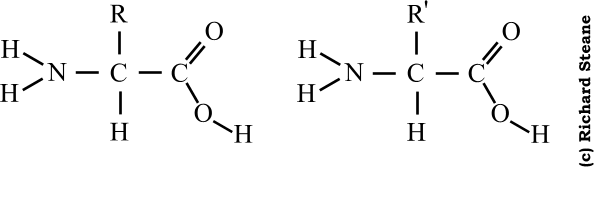
- The general formula of an amino acid can be written as,
- An amino acid is an amino carboxylic acid.
- R is the side chain or residue and it represents the group other than -NH2 and -COOH.
- It may be a hydrogen atom (H) or a methyl group (-CH3) or an aliphatic group or an aromatic group or a heterocyclic group.
- The amino acids are classified based on the nature of R groups.
- Twenty different amino acids are commonly found in proteins.
- The first to be discovered was asparagine, in 1806.
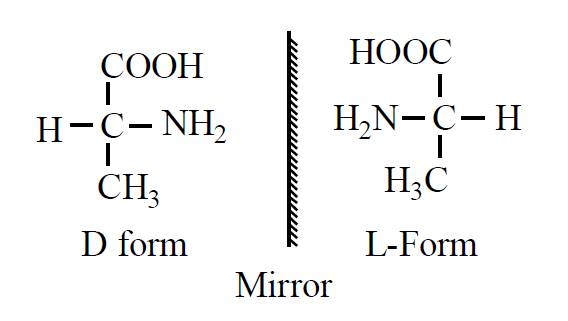
D and L amino acids
|
PROPERTIES OF AMINO ACIDS
a) Physical properties :
Amino acids are coloureless, crystalline, generally soluble in water,in acid and in alkali but sparingly soluble in organic solvents.
b) Chemical properties: Zwitterion
Due to the presence of an acidic and a basic group in the same molecule, the amino acid exists largely as a dipolar ion or a zwitterion, which can react as an acid as well as a base. In zwitterion, the proton from the carboxylic group is transferred to the amino group. Thus a zwitterion carries both positive and negative charges.
a) Physical properties :
Amino acids are coloureless, crystalline, generally soluble in water,in acid and in alkali but sparingly soluble in organic solvents.
b) Chemical properties: Zwitterion
Due to the presence of an acidic and a basic group in the same molecule, the amino acid exists largely as a dipolar ion or a zwitterion, which can react as an acid as well as a base. In zwitterion, the proton from the carboxylic group is transferred to the amino group. Thus a zwitterion carries both positive and negative charges.
CLASSIFICATION OF AMINO ACIDS
Amino acids can be divided on the basis of:
- Requirement (Essential, nonessential)
- Functional group (Basic, acidic, neutral)
- R group (Polar or non polar)
|
1. Essential and non essential proteins:
|
2. Types of amino acids (Functional group) :
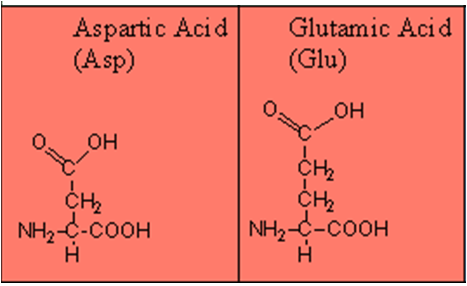
- Basic- 1 carboxyl and 2 amino groups.
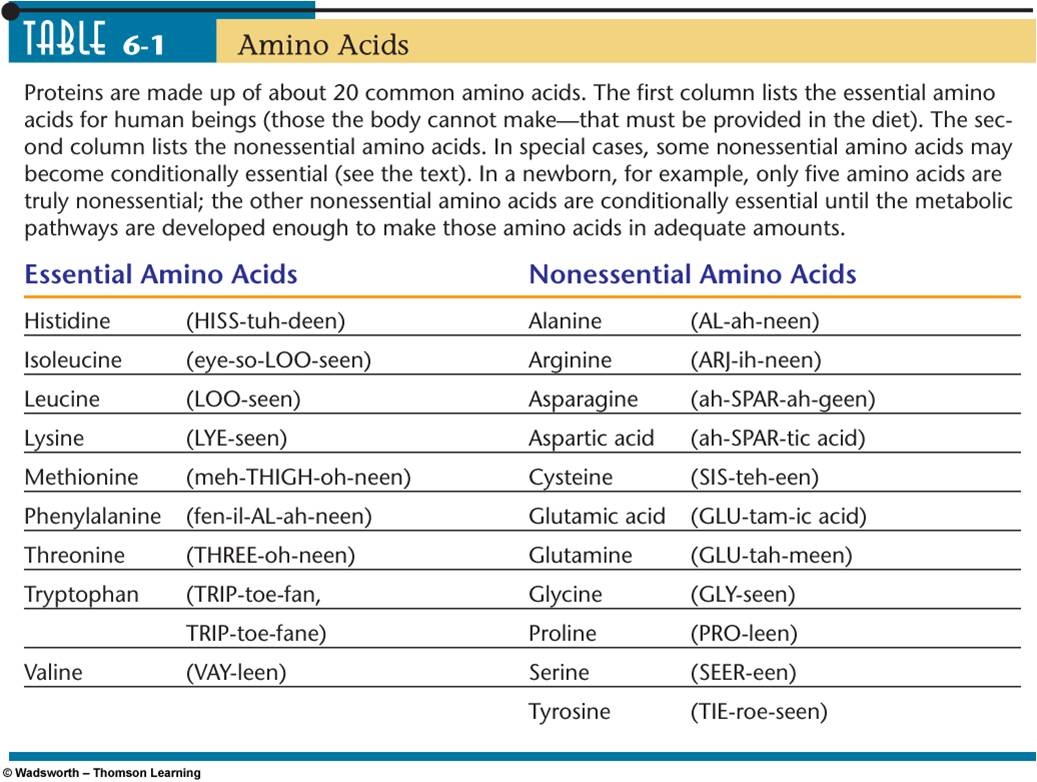
- Acidic- 2 carboxyl and 1 amino group.
- Neutral– 1 carboxyl and 1 amino group.
3.
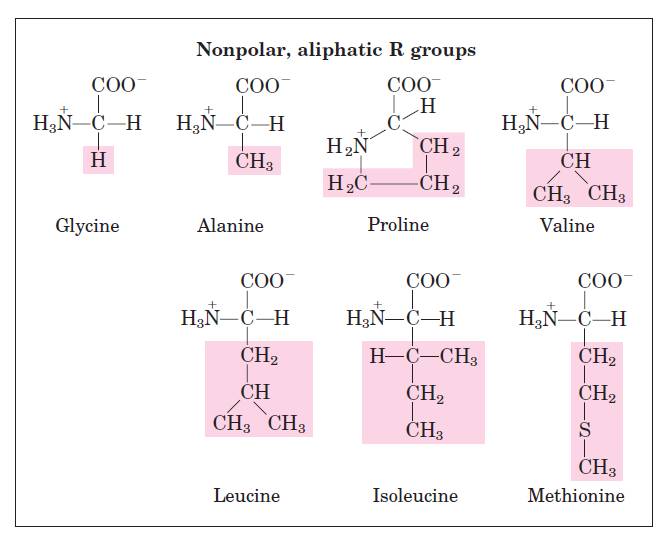
R group (Polar or non polar)
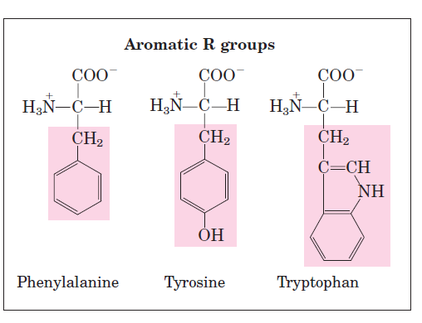
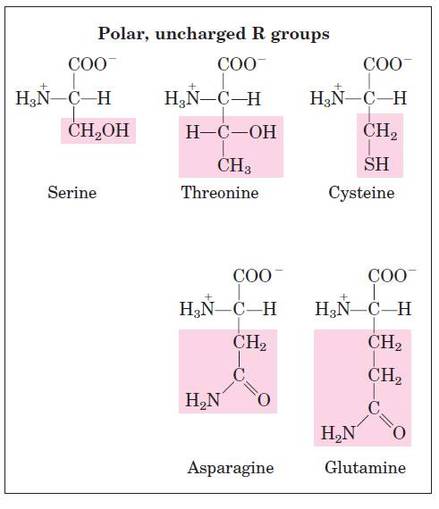
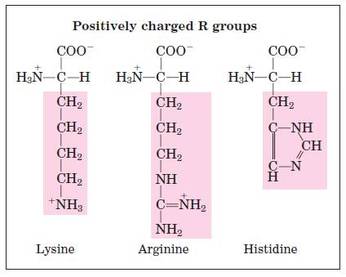
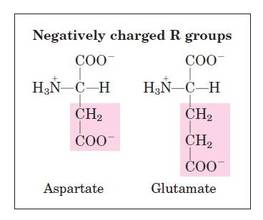
Structural formula of amino acids (20)
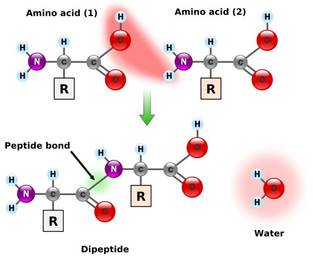
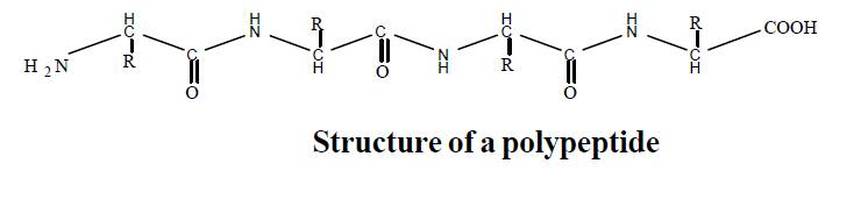
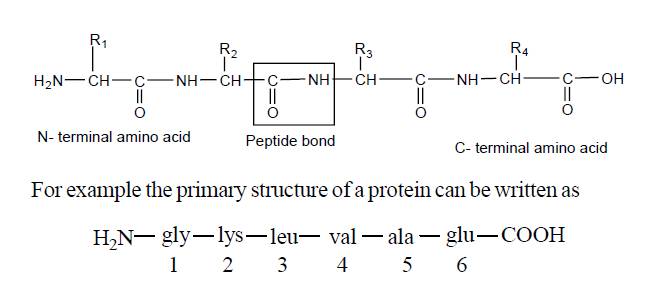
FORMATION OF PEPTIDE BOND
|
|
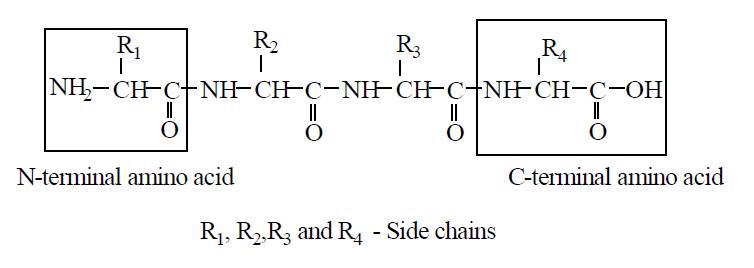
N and C terminal ends of protein
|
Functions of amino acids
- Formation of proteins
- Formation of biologically active compound like thyroxin, melanin, adrenalin, IAA, Coenzymes etc.
- Formation of glucose
- Formation of amines
- Nitrogen storage
- Antibiotics
- Biological buffers
- Cell wall of prokaryotes
Proteins
- The term coined by' Berzelius'.
- Proteins are among the most important macromolecule of the cell.
- They play a very important role in structure and metabolism of cell.
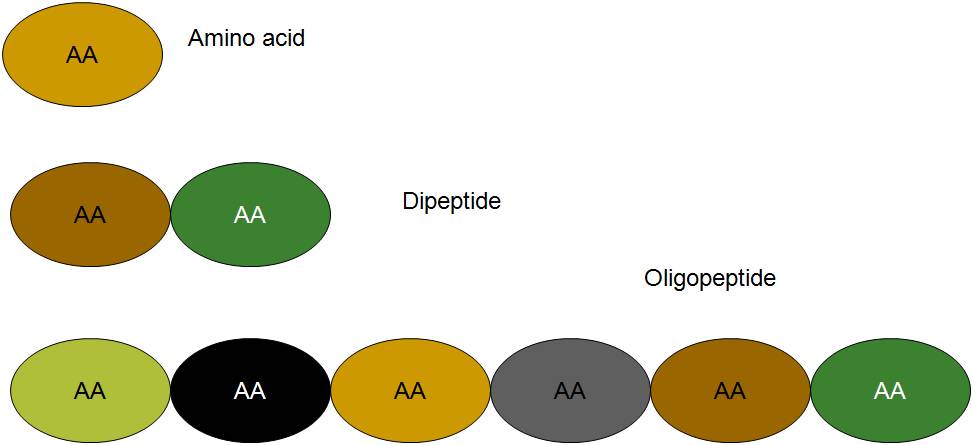
- Protein is made up of one or more polypeptide chains.
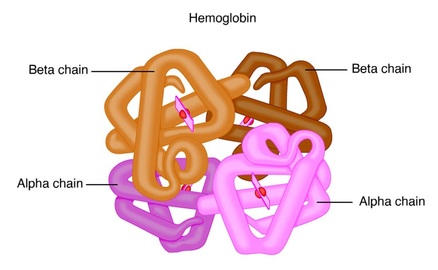
Many proteins, such as myoglobin, consist of a single polypeptide chain. Others contain two or more chains, which may be either identical or different. For example haemoglobin is formed of 4 polypeptide chains, of which two a chains are of one kind and the other two b chains are of another kind.
Properties of proteins
Physical properties
- Colour and taste :Proteins are colourless and usually tasteless. These are homogeneous and crystalline.
- Solubility: Solubility of proteins is influenced by pH. Solubility is lowest at isoelectric point and increased with increasing acidity of alkalinity.
- Optical activity: All protein solutions rotate the plane polarised light to the left i.e. these are levorotatory.
- Colloidal nature :Because of their giant size, the proteins exhibit many colloidal properties are:
- Their diffusion rate is extermely low.
- They may produce considerable light-scattering in solution, thus resulting in visible turbidity (Tyndall effect)
Chemical properties
- Hydrolysis
- By acidic agents : Proteins upon hydrolysis with concentrated mineral acids such as, HCl yield amino acids in the form of their hydrochlorides.
- By proteolytic enzymes like pepsin and trypsin.
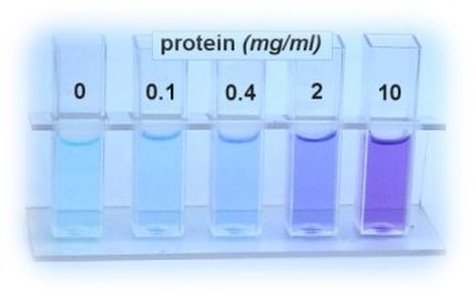
- When a protein solution is treated with alkaline CuSO4 reagent, the peptide bonds present in the protein interact with copper ions and forms violet coloured Biuret complex . The colour deepens which depend on the number of peptide bond present in the protein. All proteins except dipeptides react with Biuret reagent because a minimum of two peptide linkages are involved in this reaction.
Biuret test
STRUCTURE OF PROTEINS
Linder strom - Lang suggest four types of structural organisation for proteins.
Linder strom - Lang suggest four types of structural organisation for proteins.
- Primary structure
- Secondary structure
- Tertiary Structure and
- Quaternary strucutre
1. Primary structure
|
2. Secondary structure
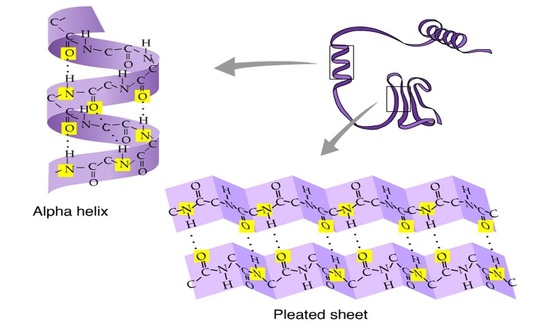
Secondary structure refers to the shape taken up by the polypeptide chain(s) within a protein molecule as the result of the formation of hydrogen bond between amino acids.
Secondary structure includes two forms
a) α-helix
b) β- pleated sheet
Secondary structure includes two forms
a) α-helix
b) β- pleated sheet
|
a) α-helix :
|
b) β- pleated sheet
|
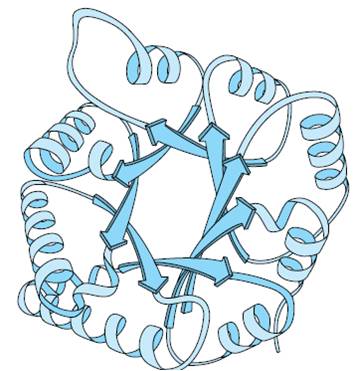
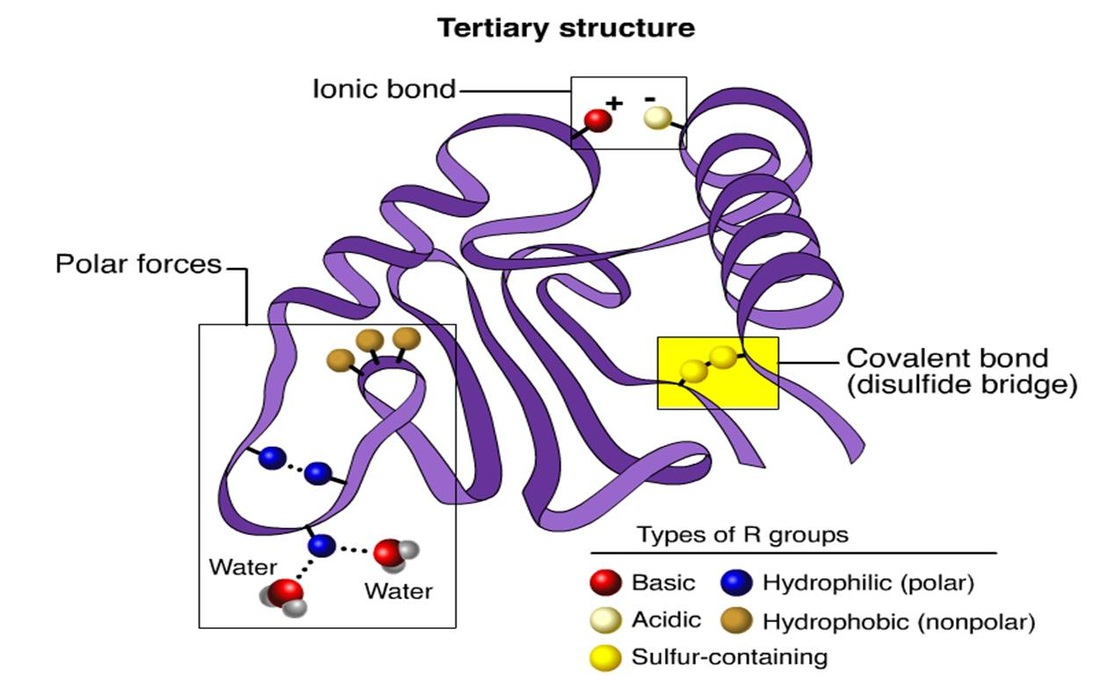
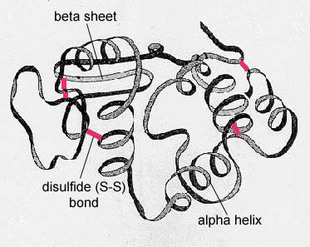
3. Tertiary structure
- The polypeptide chain with secondary structure may be further folded, super-folded, twisted about itself forming many sizes. Such a structural confirmation is called tertiary structure.
- These are common in globular proteins.
- The bonds responsible for interaction between groups of amino acids are as follows:
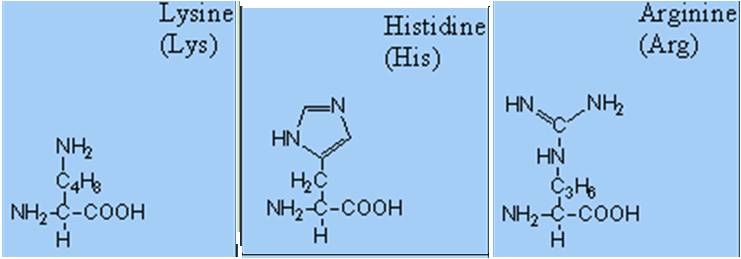
- Hydrophobic interactions :Normally occur between nonpolar side chains of amino acids such as alanine, leucine, methionine, isoleucine and phenyl alanine. They constitute the major stabilzing forces for tertiary structure forming a compact three-dimentional structure.
- Hydrogen bonds :Normally formed by the polar side chains of the amino acids.
- Ionic or electrostatic interactions:The interaction occurs between opposite charged polar side chains of amino acids, such as basic and acidic amino acids.
- Vander -wall forces : Occurs between non polar side chains.
- Disulphide bonds :These are S-S bonds formed between - SH groups of distant cysteine residues.
4. Quaternary structure
|
TYPES OF PROTEINS ( Based on shape)
On the basis of shape of the molecule, proteins can be divided into two groups
a) Fibrous proteins
b) Globular proteins
a) Fibrous proteins
b) Globular proteins
TYPES OF PROTEINS (Based on composition)
- Simple protein : These proteins on hydrolysis yield only a-amino acids. (eg). albumin, globulin
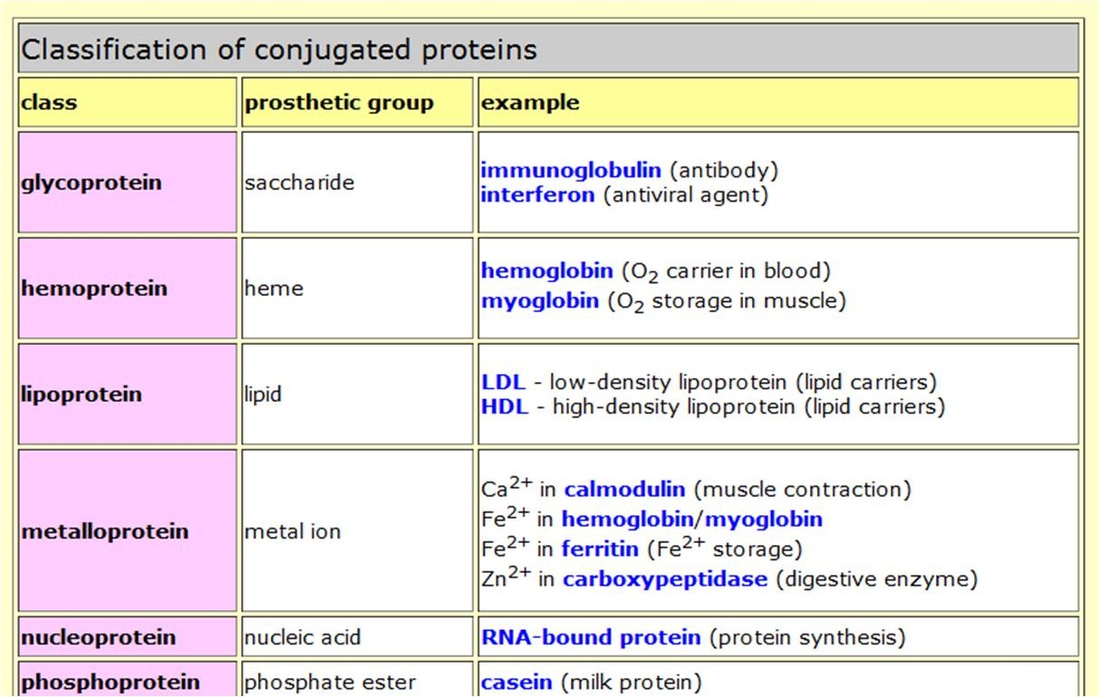
- Conjugated protein : These are proteins composed of simple proteins combined with non-protein part called as prosthetic groups.
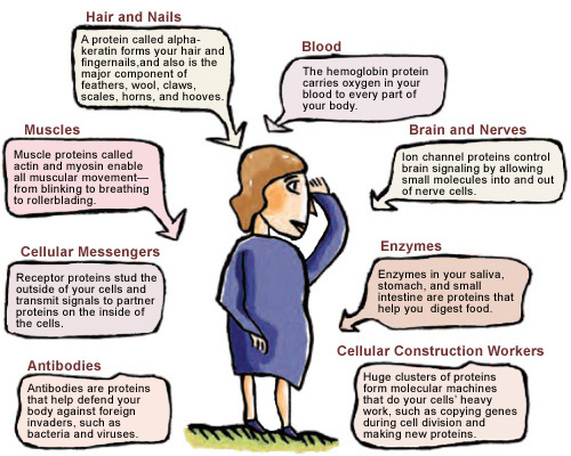
FUNCTIONS OF PROTEINS
Functional diversity of proteins
Proteins are the fundamental constituents of all protoplasm and are involved in the structure and functions of living cells. Proteins have many different biological functions.
10. Visual Pigment: Pigment rhodopsin present in the rod cells and iodopsin of cone cells of retina are proteins. These enable us to see in dark as well as in light and also differentiate colors.
Proteins are the fundamental constituents of all protoplasm and are involved in the structure and functions of living cells. Proteins have many different biological functions.
- Catalytic proteins : Enzymes are proteins which have catalytic power, far beyond that of man-made catalysts. They enhance the rate of biochemical reactions. (eg.) amylase, carbonic anhydrase etc.
- Nucleoproteins :Histones are basic proteins found in association with nucleic acids.They serve as carriers of genetic characters and hence govern inheritance of traits.
- Hormonal proteins :Some hormones are proteins which regulate numerous physiological functions. (eg) growth hormone, insulin and glucagon.
- Storage proteins :They have the function of storing amino acids as nutrients and as building blocks for the growing embryo. (eg) casein of milk, ovalbumin of egg white.
- Transport proteins :They are capable of binding and transporting specific types of molecules via blood. (eg) hemoglobin and albumin.
- Contractile proteins :Proteins such as actin and myosin in skeletal muscle function as essential elements in contractile and motile systems.
- Defensive proteins : Some proteins have protective or defensive functions. The blood proteins - thrombin and fibrinogen participate in blood clotting. Antibodies or immunoglobulins are protective proteins which prevent the onset of diseases in the body.
- Structural proteins :
- Wall of pollen grains is also proteinaceous.
- Matrix of connective tissue contains collagen, elastin and reticulin proteins in the form of fibres.
- Matrix of cartilage (chondrin) is formed of protein and the bones formed of protein ossein.
- Horny layer of skin, scales, scales, feather, hair, wool, etc are formed of keratin sulphate.
- Fibrous proteins from supporting filaments, cables or sheets and form structures like tendons and ligaments.
- Tendons are formed of collagen proteins and ligaments elastin.
- Keratin is found in hair, nails and feathers.Proteins such as collagen, keratin etc. serve as structural elements.
10. Visual Pigment: Pigment rhodopsin present in the rod cells and iodopsin of cone cells of retina are proteins. These enable us to see in dark as well as in light and also differentiate colors.