ISC 11> UNIT OF LIFE> 2. ENZYMES
Scope of syllabus
|
Enzymes: molecular structure, general properties, classification, mechanism of enzyme action, allosteric modulation, factors affecting enzyme activity.
General properties, nomenclature and classification of enzymes. Lock and key hypothesis and Induced fit theory with diagram to give a clear concept of enzyme action. Factors affecting enzyme activity. A brief idea of allosteric modulation, isozymes and zymogens. simulation- ENZYMES |
|
PRACTICAL BASED LEARNING
|
ENZYMES & LIFE PROCESSES
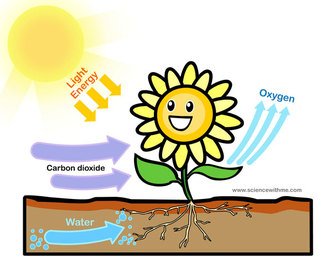
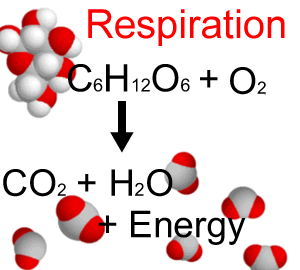
There are various biochemical activities taking place in a living cell, called metabolism. This includes building of new tissues, repair and replacement of old tissue, breakdown and conversion of food to energy, disposal of waste materials, reproduction i.e., all the activities that we characterize as "life."
Most of these biochemical reactions do not take place spontaneously. The biochemical reactions necessary for all life processes are possible because of the phenomenon of catalysis. Catalysis is defined as the acceleration of a chemical reaction by some substance which itself undergoes no permanent chemical change. The catalysts of biochemical reactions are enzymes and are responsible for bringing about almost all of the chemical reactions in living organisms. Without enzymes, these reactions take place at a rate far too slow for the pace of metabolism. In the laboratory, the average protein must be boiled for about 24 hours in a 20% HCl solution to achieve a complete breakdown. In the body, the breakdown takes place in four hours or less under conditions of mild physiological temperature and pH. |
|
HISTORY OF ENZYMES
|
Wilhelm Kühne
Eduard Buchner
James B. Sumner
|
ORGANIC AND INORGANIC CATALYST
|
LEARNING METHOD : INVESTIGATION
|
CHEMICAL NATURE OF ENZYMES............?
LEARNING METHOD : INVESTIGATION
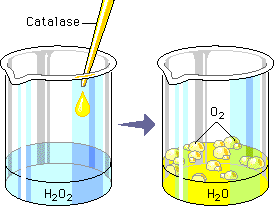
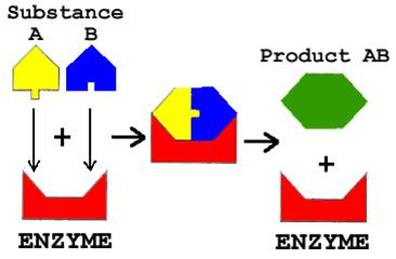
All known enzymes are proteins. They are high molecular weight compounds made up principally of chains of amino acids linked together by peptide bonds. Enzymes can be denatured and precipitated with salts, solvents and other reagents.
All known enzymes are proteins. They are high molecular weight compounds made up principally of chains of amino acids linked together by peptide bonds. Enzymes can be denatured and precipitated with salts, solvents and other reagents.
HOW DO ENZYMES WORK............?
LEARNING METHOD : INVESTIGATION
Animation- 2
http://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter2/animation__how_enzymes_work.html
http://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter2/animation__how_enzymes_work.html
ENZYMES AND ACTIVATION ENERGY…………………..?
|
LEARNING METHOD : INVESTIGATION
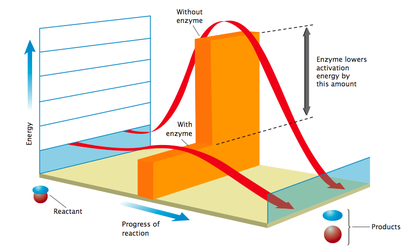
Consider a mixture of ethanol and oxygen maintained at room temperature. Although a reaction between the two substances is thermodynamically possible, it does not occur unless energy is supplied to it. This energy is called the activation energy. Thus, activation energy is defined as the energy required to make substances react. It represents the energy barrier that has to be overcome before a reaction can take place to form products.
The greater the activation energy, the slower the reaction at any particular temperature. If the activation energy of a reaction is decreased, the rate of reaction would be increased. |
Activation energy
|
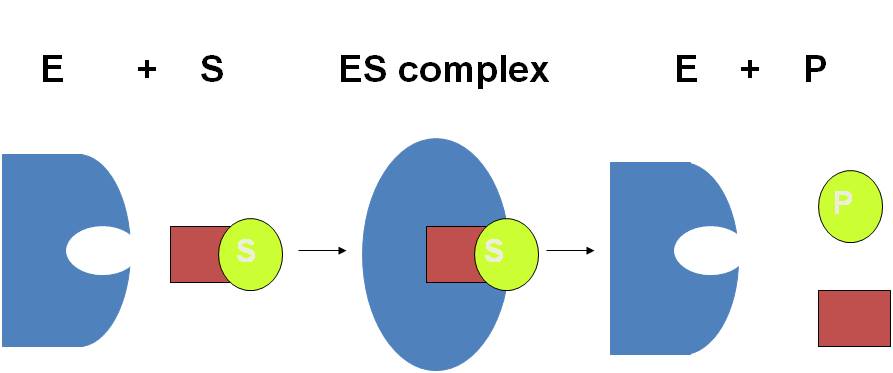
COURSE OF AN ENZYME CATALYSED REACTION
LEARNING METHOD : DATA ANALYSIS
Your browser does not support viewing this document. Click here to download the document.
THEORIES TO EXPLAIN THE ENZYME SUBSTRATE COMPLEX FORMATION:
GOOGLE SEARCH
Animation: 4
http://www.sumanasinc.com/webcontent/animations/content/enzymes/enzymes.html
Animation: 5
http://www.boardworks.co.uk/media/797600dd/AP%20Biology%20Sample/3_2_induced_fit_animation.swf
http://www.sumanasinc.com/webcontent/animations/content/enzymes/enzymes.html
Animation: 5
http://www.boardworks.co.uk/media/797600dd/AP%20Biology%20Sample/3_2_induced_fit_animation.swf
A) LOCK AND KEY THEORY
|
B) INDUCED-FIT HYPOTHESIS
|
CAN ENZYMES ACT ON MORE THAN ONE SUBSTRATE.............? (Specificity)
INVESTIGATION
Your browser does not support viewing this document. Click here to download the document.
FACTORS AFFECTING ENZYME ACTION
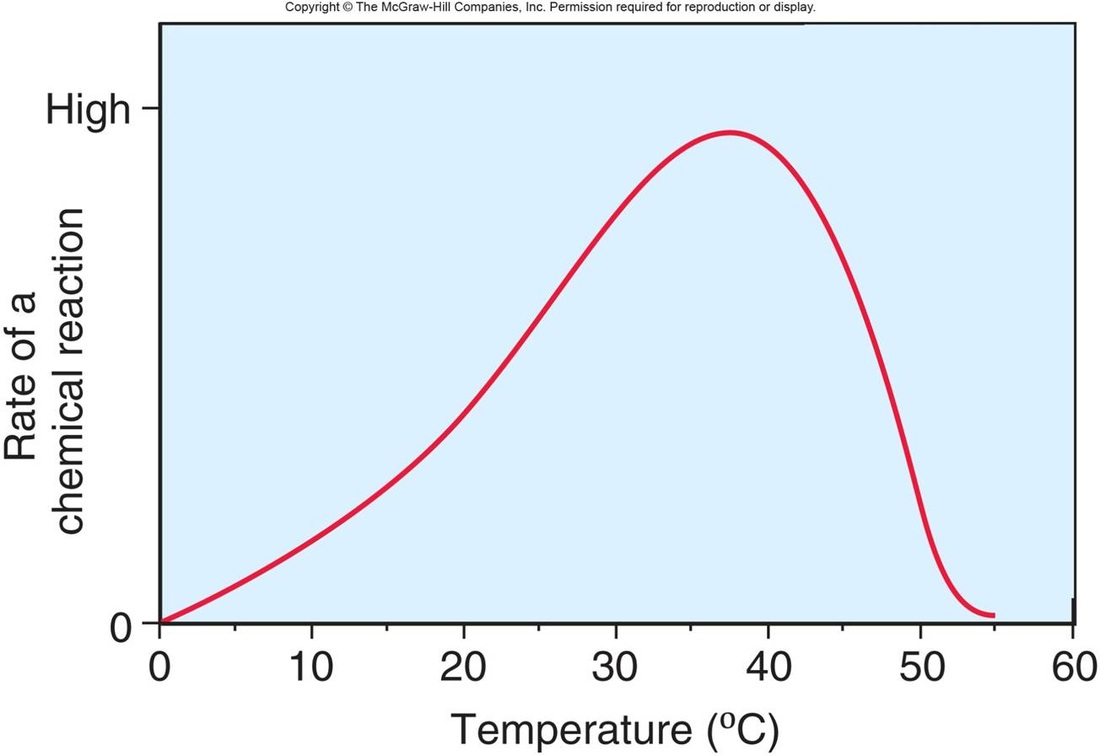
1. ENZYME AND TEMPERATURE
|
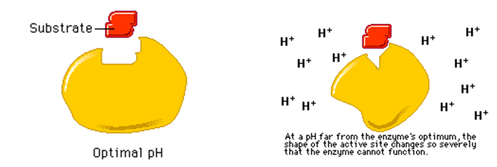
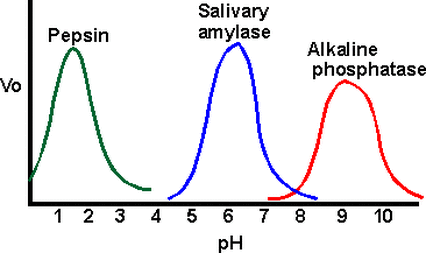
2. ENZYME AND pH
- The optimum pH is the pH at which the rate of an enzyme catalyzed reaction is at its maximum.
- At this pH, the intramolecular bonds which maintains the structure of the enzyme, are intact, the conformation of the active site is most ideal for binding of substrate and the frequency of successful collision between enzyme and substrate molecules is the highest.
- At pH lower or higher than the optimum, the concentration of hydrogen ions would have changed and this would alter the charges on R groups of the amino acids residues of the enzymes.The ionic bonds would be disrupted and the binding of substrate would be affected.
- Enzymes work within a narrow range of pH. If the pH is altered by a small extent from the optimum pH, the effects are normally reversible.If the pH is altered by a large extent, the conformation of the enzyme molecule would be severely affected and denaturation of the enzyme might be irreversible.
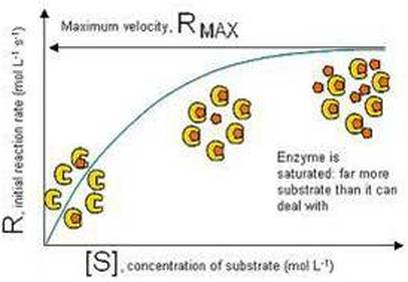
3. ENZYMES AND VARYING SUBSTRATE CONCENTRATION
|
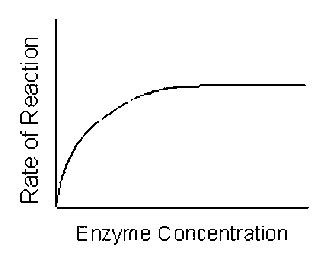
4. EFFECTS OF VARYING ENZYME CONCENTRATION
|
5. INHIBITION OF ENZYME ACTION
An inhibitor is a substance that prevents an enzyme from catalysing its reaction. It decreases the rate of an enzyme catalysed reaction. It does so by combining with the enzyme to form an enzyme-inhibitor complex, which then cannot combine with the substrate molecule.
There are three different ways by which the action of an enzyme can be inhibited
- Competitive inhibition
- Noncompetitive inhibition
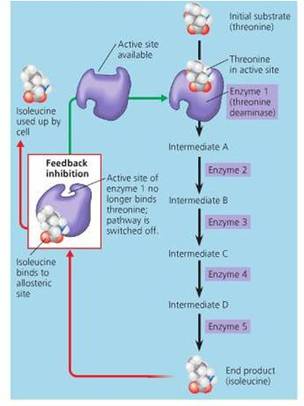
- Allosteric or feed back inhibition
|
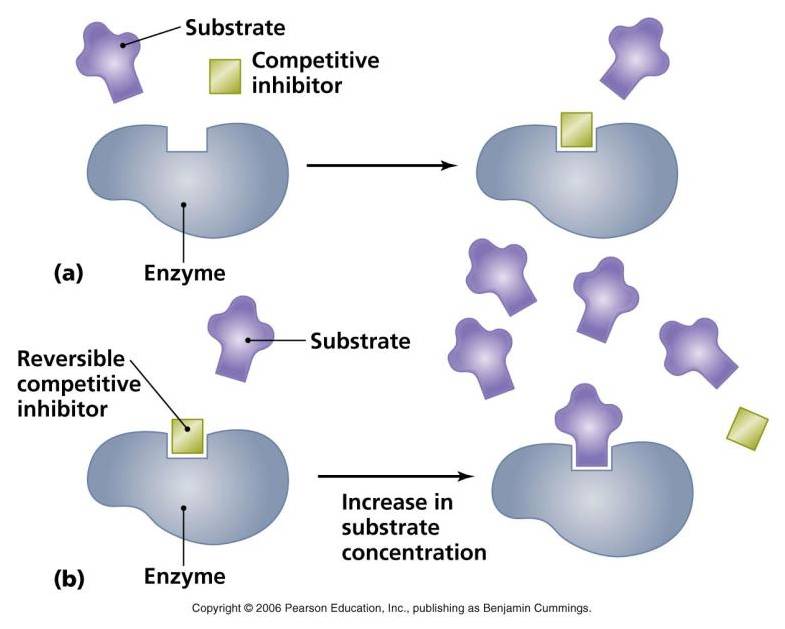
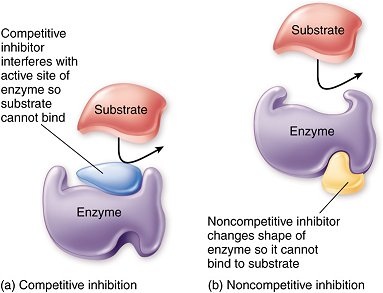
A. Competitive inhibition
|
|
B. Non-competitive inhibition
|
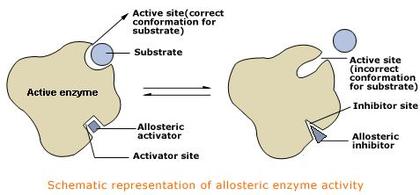
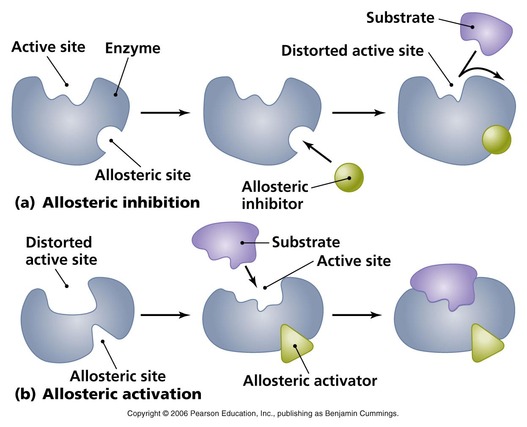
C. Allosteric inhibition
- Allosteric enzyme is one whose activity can be altered by molecules acting at a site other than the active site. This site is called the allosteric site. The binding of a regulatory molecule to the allosteric site changes the overall shape of the enzyme. This can either enable or prevent the binding of substrate to the active site.
- There are two types of regulatory molecules, namely allosteric activators and allosteric inhibitors. .
- Allosteric activators enable the substrate to bind to the active site and thus increase the rate of reaction.
- In contrast, allosteric inhibitors prevent the binding of the substrate to the active site and therefore decrease the rate of reaction.
|
ENZYMES CLASSIFICATION
CLASSIFICATION OF ENZYMES ON THE BASIS OF CHEMICAL COMPOSITION:
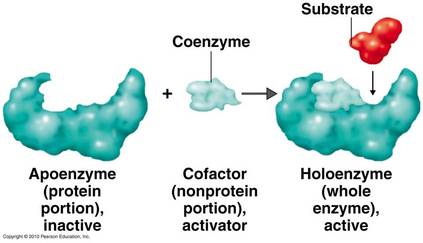
ENZYMES CO-FACTORS
Based on their composition, enzymes are of two types :
a) Simple enzymes:
These are simple protein molecules containing amino acids only. e.g. pepsin, trypsin, urease, amylase etc.
b) Conjugated enzymes
Most enzymes require non-protein components called cofactors for their activities.
These may vary from simple inorganic ions to complex organic molecules.
a) Simple enzymes:
These are simple protein molecules containing amino acids only. e.g. pepsin, trypsin, urease, amylase etc.
b) Conjugated enzymes
Most enzymes require non-protein components called cofactors for their activities.
These may vary from simple inorganic ions to complex organic molecules.
|
The three types of cofactors that exist are stated below:
|
| pbl_enzyme.pdf | |
| File Size: | 973 kb |
| File Type: | |